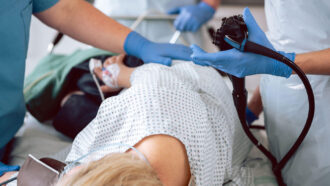
A recent study reported a smaller-than-expected benefit from screening colonoscopies. But the study has important caveats, gastroenterologists say, making it ripe for misinterpretation if that context isn’t included.
The study was the first randomized controlled trial — widely considered the gold standard for testing medical interventions — of the procedure. Published online October 9 in the New England Journal of Medicine, the study followed participants invited to have a colonoscopy and compared how they fared with participants who weren’t invited to undergo the procedure. The risk of colorectal cancer at 10 years was reduced by 18 percent in the invited group. But there wasn’t a meaningful difference in the risk of death from colorectal cancer between the two groups, the study reported.
This was disappointing, gastroenterologists say, as past research has shown screening colonoscopies to be more effective in reducing the risks of developing and dying from colorectal cancer. Those previous data were from observational studies, which don’t randomly assign patients to get, or not get, a treatment.
But a closer look at the details of the new study reveals why it shouldn’t be interpreted as a slam-dunk against the screening test. First, fewer than half of the people invited to have a colonoscopy actually did. The study also didn’t follow patients long enough to fully assess the risk of death from colorectal cancer. And some of the physicians who did the procedure didn’t meet a minimum quality benchmark.
These issues limit what this study can tell us about screening colonoscopies. On top of that, this study should not be used to cast doubt on colorectal cancer screening in general, says Folasade May, a gastroenterologist and health services researcher at UCLA Health. “Screening is effective, and it saves lives,” she says. “We have enough data to promote screening.”
Screening importance
Colorectal cancer is the second-leading cause of cancer deaths for men and women combined, according to the American Cancer Society. It’s expected to kill more than 52,000 Americans in 2022. There are racial disparities in who gets and dies from the disease. Rates of incidence and death are 21 percent and 44 percent higher in Black men compared with white men; the rates for Black women compared with white women are 18 percent and 31 percent higher, respectively.
The U.S. Preventive Services Task Force recommends screening for colorectal cancer in adults ages 45 to 75 years old (SN: 5/31/18). There are different screening options, including stool-based tests; colonoscopy, which examines the whole colon; and sigmoidoscopy, which looks at a portion of the colon. Average-risk individuals — those who don’t have a family history of colorectal cancer or other conditions that increase risk — can choose the option that works for them. “We just want people to get screened,” says gastroenterologist Sophie Balzora of the New York University Grossman School of Medicine. “The best test is the one that gets done.”
The fecal immunochemical test, or FIT, and colonoscopy are commonly performed in the United States. The FIT detects tiny amounts of blood in the stool, which can be a sign of colorectal cancer, and is done at home.
During a colonoscopy, a physician looks for and removes polyps, growths of tissue that can become cancerous. But the procedure’s expense, time and preparation can be prohibitive for some patients, says Carol Burke, a gastroenterologist at the Cleveland Clinic. People may not have the flexibility to take time off work for the procedure or have someone who is available to drive them home, for example. To complete a colonoscopy, “you have to be sure that you can address the patient’s barriers,” Burke says.
Important caveats
The potential barriers to getting a colonoscopy mean it’s not enough to just tell someone to do it. That’s also the case in Poland, Norway and Sweden, where colonoscopies are not commonly used to screen for colorectal cancer. One-third of roughly 84,000 study participants from these countries were invited to get colonoscopies. The other two-thirds made up the “usual care” group. But “the intervention was an invitation, not a colonoscopy,” Balzora says. Only 42 percent of the participants invited to get the procedure had one. The majority of the invitees turned the invitation down.
“If you don’t actually have the test, it can’t possibly protect you,” says gastroenterologist Aasma Shaukat of the New York University Grossman School of Medicine.
Another limitation of the new study has to do with time. Colon cancer develops slowly. Most polyps don’t become cancerous, but for those that do, it can take 10 years or more. Then it takes time for the cancer to spread and become fatal. At least 15 years of follow up are needed to really look at the impact on colorectal cancer deaths, Shaukat says, so the study’s report at 10 years isn’t long enough.
And the quality of the colonoscopies performed in the study varied. One standard is the adenoma detection rate, the number of colonoscopies that turn up a precancerous polyp, or adenoma, divided by the number of colonoscopies performed over a period of time. In the new study, nearly 30 percent of the physicians doing the procedures had rates below the recommended minimum quality rate.
In their paper, the study’s authors acknowledge these limitations. They note that the colonoscopy-by-invitation approach may have underestimated the benefits of the procedure. They say that reductions in risk of cancer are expected to appear before reductions in risk of death; the team will report results again at 15 years of follow-up. And, they add, differences in quality benchmarks among practitioners may have affected the detection of cancer.
The new study needs to be considered among other evidence for the effectiveness of screening colonoscopies, Shaukat says. For example, an analysis that combined observational studies of colonoscopy, published in 2014 in the British Medical Journal, reported that the procedure reduces both colorectal cancer incidence and mortality by close to 70 percent.
Another observational study looked at an organized screening program that used colonoscopy, sigmoidoscopy and FIT. The program led to a boost in screening that was linked to a 25 percent decrease in the annual incidence of colorectal cancer from 2000 to 2015 and a 52 percent drop in deaths from the cancer, researchers reported in Gastroenterology in 2018.
There is also a randomized controlled trial going on now in the United States that will compare head-to-head the effectiveness of screening with colonoscopy or FIT in average-risk people. So there’s more data to come. The new study “isn’t the end-all, be-all study,” May says. “We haven’t closed the door on colonoscopy.”

 A new treatment could restore some mobility in people paralyzed by strokes
A new treatment could restore some mobility in people paralyzed by strokes  What has Perseverance found in two years on Mars?
What has Perseverance found in two years on Mars?  This robot automatically tucks its limbs to squeeze through spaces
This robot automatically tucks its limbs to squeeze through spaces  Greta Thunberg’s new book urges the world to take climate action now
Greta Thunberg’s new book urges the world to take climate action now  Glassy eyes may help young crustaceans hide from predators in plain sight
Glassy eyes may help young crustaceans hide from predators in plain sight  A chemical imbalance doesn’t explain depression. So what does?
A chemical imbalance doesn’t explain depression. So what does?