In the theater of the brain, nerve cells have long been cast as the stars, bringing mental scenes to life with their electrical and chemical performances. Yet many of the cellular actors in the human brain are glial cells, presumably the supporting cast and behind-the-scenes stagehands.
In recent decades, though, accumulating evidence has shown that glia are not just minor players that keep the show running. They actually play starring roles in many of the brain’s most important acts, such as remembering, learning and thinking.
And the latest research points to a surprising new setting for the story of glia: outside the brain. Mysterious populations of glia reside in the heart, spleen, lungs and various other organs. But no one knows how they’ll fit into the plot. Early hints suggest the story is going to be riveting.
Already, tantalizing clues are rolling in about what these cells are doing. Glia appear to help regulate the heart’s beating, for instance. Glia in the spleen reside right between nerve cells and immune cells — a perfect spot to influence the connection between health and stress. Exactly what glia are up to in the lungs is unknown, but whatever it is seems important, early experiments suggest — mice with no lung glia die.
“The fact that now there are these new glial cell populations being discovered in unique organs will hopefully trigger a lot of lightbulbs,” says Sarah Ackerman, a neurobiologist at Washington University in St. Louis. Like most researchers who study glia, Ackerman focuses on glia inside the brain.
She sees big potential in the handful of new studies that look at far-flung glia. “There’s going to be a revelation that across all of these organs, there are specialized glia that are supporting the function of the neurons there, but also overall organ health.”
Understanding the roles of glia outside the brain could have big implications for human health, leading to better ways to treat heart disorders, immune system problems and even lung cancer, some scientists suspect.
“If we continue to ignore these cells, it’s only going to slow us down,” says Tawaun Lucas, a neuroimmunologist at Genentech in San Francisco. He recently uncovered details about glia in mouse spleens.
It’s too early to know how important glia outside the brain will turn out to be. Maybe these studies on glia represent a burgeoning new field of research. Or maybe the mystery is never solved. “The connection it’s going to have to the conventional world of glia as we know it remains to be seen,” says Bruce Ransom, a neurologist and neuroscientist at the City University of Hong Kong who is also an editor in chief of the scientific journal Glia.
Still, the potential plotlines of these newly described groups of glia are worth following. “We’re always looking for that little opening that you can enlarge and see something really important,” Ransom says. “That’s a possibility here.”
Immune system meets nervous system
Glia are named for glue, the sticky substance that exists solely to hold other, more important things together. In the brain, various glia do lots of supporting. Glia called astrocytes keep nerve cells fed. Microglia combat invaders. And oligodendrocytes insulate nerve wires with a fatty substance called myelin. But glia are now known to have much fancier jobs too, including changing the signals that pass between nerve cells, guiding the growth of nerve cells and pruning neural connections called synapses (SN: 8/22/15, p. 18).
Researchers have a good handle on the roles of some glia outside the brain. Enteric glia help the gut digest food, for instance, and a type of glia called Schwann cells, sisters to the brain’s oligodendrocytes, spread myelin on peripheral nerves to help speed signals along. In the skin, specialized Schwann cells kick off pain sensations, scientists reported in Science in 2019. Less is known about glia in other organs, such as the spleen glia that intrigue Lucas. Naming these glia can be tricky, since the cells sometimes share similarities with multiple types of other glia. For now, these outsider glia are often lumped into one of two catchall categories, nonmyelinating Schwann cells or satellite glia.
Lucas started out as a neuroscientist at Stanford University, focusing on the brain. Then a mouse under a microscope shifted his attention. The mouse was genetically engineered so that its glia glowed green. As Lucas looked throughout the body, he saw green cells all over, including in the spleen, lymph nodes, kidney, liver and lungs.
His adviser at Stanford, neurologist Marion Buckwalter, was quickly captivated by the spleen glia. “I started reading about the spleen, and I thought, ‘This is really fascinating,’ ” she says. The spleen is packed with immune cells. Like many organs, the spleen also contains nerves that are part of the body’s sympathetic nervous system. This control system can dilate pupils, quicken heart rates and get the body sweating. Often called the “fight-or-flight system,” the sympathetic nervous system flies into action under threats, says Lucas, “whether you get kicked in the head, or are chased by a tiger or have stressful things going on at work.”
The sympathetic nervous system and the immune system converge in the spleen, and glia may be particularly important in this connection. Experiments with the mice revealed big, complex glia in the spleen right alongside message-sending nerve cell axons. And “just microns away, there are immune cells,” Buckwalter says. The spleen’s glia are perfectly positioned to communicate between the nervous system and the immune system, the researchers reported in 2021 in Glia.
By looking at the genetic instructions inside those glia found in the spleen, Lucas and colleagues showed that the spleen’s glia have the cellular equipment to speak the language of both immune cells and nerve cells: They can sense chemical signals made by immune cells and they can sense chemical signals sent by nerve cells. How these cells use this machinery is unknown, but the results position spleen glia to be important communicators. Until Lucas’ experiments, no one had any clues about the glia’s behavior, Buckwalter says.
The possibility of communication between glia, nerve cells and immune cells in the spleen “has potentially huge implications,” Buckwalter says. Stress and brain injuries such as strokes can harm the immune system. After a stroke, for instance, the sympathetic nervous system instigates a die-off of immune cells called B lymphocytes in the spleen, which can lead to dangerous infections. Immune cells also play a role in autoimmune disorders such as multiple sclerosis and rheumatoid arthritis, which can flare up in people who are stressed. If glia influence the immune cells, perhaps their behavior can be tweaked to prevent such flare-ups.
Research so far is preliminary. It’s not yet clear whether these spleen glia are in fact sending messages between the nervous system and the immune system, and if so, what the results of those conversations are. It’s too soon to say with any certainty that glia in people’s spleens are involved in autoimmune disorders, Buckwalter says. But the idea has piqued her interest.
Studying these mysterious cells in organs feels like a different sort of science, she says. It’s harder than just trying to find a missing piece of a puzzle. “It’s like we just got a puzzle, and the pieces aren’t labeled and the box has no picture on it.”
The beating heart
A similar puzzle can be found in the heart.
Cody Smith, who studies neural biology at the University of Notre Dame in Indiana, was well aware that the heart was packed with nerve cells. Most nerve cells are thought to have glia nearby, and Smith was eager to find the heart’s glia. Along with graduate student Nina Kikel-Coury, Smith went searching for them. “We really had no idea what to expect,” he says.
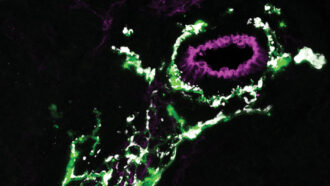
It turned out that a population of glia resides in the hearts of zebrafish. Named cardiac nexus glia, these cells appear early in zebrafish development and go on to spread out and form a gossamer-thin web around the heart, Smith and Kikel-Coury reported last year in PLOS Biology.
In zebrafish, at least, these glia do important work: They guide the development of the heart and regulate heartbeat. When the researchers messed with the cells, the fish’s heart rates increased. That’s an unusual effect, Smith says. Usually, disruptions to heart cells cause heart rates to slow.
By looking at other datasets that catalog the genes active in mouse and human heart cells, the researchers found cells that had similar collections of active genes to the heart glia in the zebrafish. The shared genes suggest that these glia may be in many species’ hearts. That data “support the idea that they’re in humans,” Smith says.
Some congenital heart disorders have defects in the outflow track of the heart, where blood exits to an artery. That’s also the spot that these glia first appear early in zebrafish development. It’s possible, Smith says, that these glia may be involved in human heart trouble. “We’re at such an early phase of this work,” he cautions. “I wish we had more answers.”
The plot thickens
Investigations of glia in the spleen and brain are just the beginning. Organs throughout the body may rely on glia, other research hints. Glia have turned up in the lungs, for instance, found there in mice by Buckwalter and Gabriela Suarez-Mier, a graduate student when the work was done.
Lung glia may be involved in breathing and oxygen exchange in capillaries, Lucas says, but details are slim. Those glia are crucial, Lucas’ preliminary experiments suggest: When researchers use a toxin to kill the glia in the lungs, “all of the animals die.”
In his current work, Lucas is studying glia’s potential role in cancer. Tumors in lungs (and also in the liver and pancreas) have nerves that attach to them, and glia wrap themselves around those nerves, Lucas says. In a lab dish, other researchers have found, Schwann cells will grow toward cancer cells. So the behavior of glia that linger near a tumor is now an important question, Lucas says.
Understanding the body’s glia and possibly enlisting them to fight disease will require more than a surface-level understanding. Techniques that reveal how individual cells use different collections of genes promise a deeper view of glia in the body. Other genetic labeling methods offer a way to monitor these mystifying glia in living animals. “The tools are going to allow us to go from, ‘OK, they’re there,’ to ‘What are they doing?’ ” says Ackerman, the neurobiologist at Washington University.
Glia biologists looking beyond the brain are still very much at the beginning of their script. “What cells are here and how do they work together?” Lucas asks. “We’re starting from square one.”
Being outside the brain puts these glia in a good position to be possible targets for therapies, Ackerman says. The brain is hard to reach with drugs, as it’s ensconced in its protective blood-brain barrier. Brain cells are also less able to repair themselves than cells in the peripheral nervous system. “We might be able to affect positive change in a more efficient way than trying to direct repair in the brain or the spinal cord,” she says.
It’s too soon to say whether glia outside the brain are part of the same story as the brain’s glia. The variety of glia residing outside the brain might all end up being individual bits of disparate biology, never coalescing into a plot that’s relevant for the entire nervous system, says Ransom, of the City University of Hong Kong. “I think it’s exciting and interesting work, and I think it’s completely justified to study it entirely,” he says. For now, there’s no telling where glia’s story will take us.

 A new treatment could restore some mobility in people paralyzed by strokes
A new treatment could restore some mobility in people paralyzed by strokes  What has Perseverance found in two years on Mars?
What has Perseverance found in two years on Mars?  This robot automatically tucks its limbs to squeeze through spaces
This robot automatically tucks its limbs to squeeze through spaces  Greta Thunberg’s new book urges the world to take climate action now
Greta Thunberg’s new book urges the world to take climate action now  Glassy eyes may help young crustaceans hide from predators in plain sight
Glassy eyes may help young crustaceans hide from predators in plain sight  A chemical imbalance doesn’t explain depression. So what does?
A chemical imbalance doesn’t explain depression. So what does?