
Worldwide, nearly half of new HIV infections among adults in 2019 occurred in women. Yet a long list of obstacles has kept many women from taking advantage of medicine that can prevent an HIV infection.
Recent, promising news about a different HIV prevention regimen could help. A long-acting injection of an HIV drug given once every eight weeks was safe and more effective in preventing infection in women than a daily pill of two HIV drugs, a large clinical trial found.
“This is incredibly exciting,” says infectious disease physician Bisola Ojikutu of Brigham and Women’s Hospital and Massachusetts General Hospital in Boston, who was not involved in the trial. If the injection is approved by the U.S. Food and Drug Administration, she says, women will have the option of a discretely administered HIV prevention drug that doesn’t require daily attention.
Although women make up a minority of those newly diagnosed with HIV in the United States, their HIV-related death rate, 5.4 per 1,000 people with diagnoses, was higher than that of males (4.5 per 1,000) or transgender women (4.3 per 1,000) in 2017, researchers report November 20 in Morbidity and Mortality Weekly Report.
Women face many barriers when it comes to protecting themselves against an HIV infection. A woman’s HIV risk is tied not only to measures within her control, but also to the amount of HIV transmission in her community, whether she has reliable health care and whether she lives in poverty or experiences intimate partner violence (SN: 11/15/19). But studies have found that women at high risk due to societal factors tend to underestimate their individual risk.
Most cisgender women acquire HIV during heterosexual contact; women have twice the risk that men do of contracting HIV during vaginal sex with an infected partner. Condoms are a helpful HIV prevention tool but their use is not fully under a woman’s control. Medicine to prevent an infection in an HIV-negative person at risk, called pre-exposure prophylaxis or PrEP, was approved in the United States in 2012. Taken consistently, the daily pill form of PrEP can lower the risk of getting HIV during vaginal sex by up to 90 percent, and as much as 99 during anal sex.
Even if a woman knows she’s at heightened risk for HIV, she may not know about PrEP. Awareness and use of the PrEP pill, called Truvada, in the United States remains low among women; they were only 6 percent of the more than 100,000 U.S. users in 2017, according to a 2018 Annals of Epidemiology study of a national prescription database. And for women taking the PrEP pill, sticking to the daily routine can be challenging, due to side effects from the medication or because of stigma, studies have found.
“Most of my female patients living with HIV have told nobody,” says Ojikutu. That stigma can extend to the PrEP pill. Taking a daily medicine to prevent HIV can appear to others as if the person is getting treatment for HIV.
Fears that family and friends would assume they were HIV-positive or that a partner would accuse them of infidelity were among those raised about PrEP by women in the Washington D.C. area, researchers reported in the Journal of AIDS Clinical Research in 2017. Adolescent girls and young women in Zimbabwe and South Africa were also worried about being thought HIV-positive or being called promiscuous if they took PrEP, according to a March 2020 study in the Journal of the International AIDS Society.
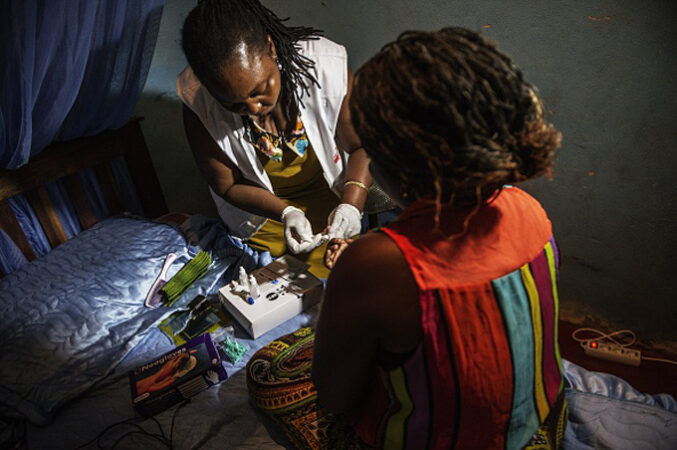
Having a more discreet way to take PrEP — such as an infrequent injection — could be appealing for many women. “It’s private,” says infectious disease physician Mina Hosseinipour of the University of North Carolina School of Medicine in Chapel Hill. “This just allows [women] a lot of autonomy.”
In the Phase III trial, which began in 2017, Hosseinipour and her colleagues enrolled more than 3,000 HIV-negative, sexually active cisgender women ages 18 to 45 in east and southern Africa. In this region, women accounted for 61 percent of new HIV infections among those 15 and older in 2019, with young women making up 73 percent of new infections among 15- to 24-year-olds, according to UNAIDS. These women are not always able “to advocate for themselves and their prevention needs,” because of their societal position and vulnerability, says Hosseinipour, who has lived and worked in Malawi, in southeastern Africa, since 2001.
The trial participants were randomly assigned to receive an injection of the HIV drug cabotegravir once every eight weeks and a daily placebo pill or a placebo injection on the same schedule and a daily PrEP pill.
Of the 38 women who became infected with HIV, four were getting the cabotegravir injection and 34 were taking the PrEP pills, the National Institutes of Health announced on November 9. That’s an HIV incidence rate of 0.21 percent in the injection group and 1.79 percent in the pill group. These interim results were so good — the injection reduced infections by 89 percent compared with the pill — that the safety board monitoring the trial recommended participants be told which medicine they are getting, so that women have the option to switch to the injection. The investigators will continue to follow trial participants to assess long-term safety.
The promising results follow those reported for a trial of cisgender men and transgender women who have sex with men; the injections reduced infections by 66 percent compared with daily PrEP pills in that trial, researchers reported in July 2020 at the International AIDS Conference.
Having a new PrEP option doesn’t change the fact that more outreach on HIV prevention directed at women needs to happen, through public health campaigns and community engagement, says Ojikutu. And as for doctors, asking about HIV risk “needs to be part of our routine engagement with patients,” she says. “It needs to be an ongoing interaction” to understand “the determinants of risk in each woman’s life.”

 Three Truths to Address Sexual Exploitation, Abuse & Harassment in the UN
Three Truths to Address Sexual Exploitation, Abuse & Harassment in the UN  COP27 Fiddling as World Warms
COP27 Fiddling as World Warms  UN chief highlights crucial role of G20 in resolving global crises
UN chief highlights crucial role of G20 in resolving global crises  Somalia: Human rights chief decries steep rise in civilian casualties
Somalia: Human rights chief decries steep rise in civilian casualties  Ukraine: UN convoy delivers vital aid to residents of Kherson
Ukraine: UN convoy delivers vital aid to residents of Kherson  COP27: Week two opens with focus on water, women and more negotiations on ‘loss and damage’
COP27: Week two opens with focus on water, women and more negotiations on ‘loss and damage’  A new treatment could restore some mobility in people paralyzed by strokes
A new treatment could restore some mobility in people paralyzed by strokes  What has Perseverance found in two years on Mars?
What has Perseverance found in two years on Mars?  This robot automatically tucks its limbs to squeeze through spaces
This robot automatically tucks its limbs to squeeze through spaces  Greta Thunberg’s new book urges the world to take climate action now
Greta Thunberg’s new book urges the world to take climate action now