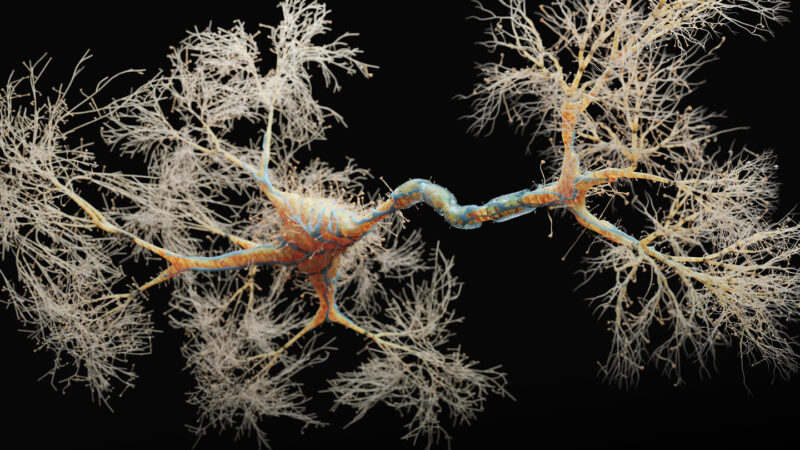
Learning lots of new information as a baby requires a pool of ready-to-go, immature connections between nerve cells to form memories quickly. Called silent synapses, these connections are inactive until summoned to help create memories, and were thought to be present mainly in the developing brain and die off with time. But a new study reveals that there are many silent synapses in the adult mouse brain, researchers report November 30 in Nature.
Neuroscientists have long puzzled over how the adult human brain can have stable, long-term memories, while at the same time maintaining a certain flexibility to be able to make new memories, a concept known as plasticity (SN: 7/27/12). These silent synapses may be part of the answer, says Jesper Sjöström, a neuroscientist at McGill University in Montreal who was not involved with the study.
“The silent synapses are ready to hook up,” he says, possibly making it easier to store new memories as an adult by using these connections instead of having to override or destabilize mature synapses already connected to memories. “That means that there’s much more room for plasticity in the mature brain than we previously thought.”
In a previous study, neuroscientist Mark Harnett of MIT and his colleagues had spotted many long, rod-shaped structures called filopodia in adult mouse brains. That surprised Harnett because these protrusions are mostly found on nerve cells in the developing brain.
“Here they were in adult animals, and we could see them crystal clearly,” Harnett says. So he and his team decided to examine the filopodia to see what role they play, and if they were possibly silent synapses.
The researchers used a technique to expand the brains of adult mice combined with high-resolution microscopy. Since nerve cell connections and the molecules called receptors that allow for communication between connected cells are so small, these methods revealed synapses that past research missed.
The team looked for the typical signs of a silent synapse: the presence of a type of receptor called NMDA and the absence of others, known as AMPA receptors. Both of these types of receptors respond to the chemical messenger glutamate, but both typically need to be present for a synapse to be active.
Of the more than 2,000 synapses that the team looked at, about 30 percent were filopodia and, of those, nearly all had characteristics suggesting that they could be silent synapses.
To test whether the connections were truly silent, the researchers turned to glutamate. Artificially adding the chemical messenger was not enough to activate the synapses, the team found, suggesting that the connections were actually silent ones.
Adding an electrical current in addition to glutamate turned these connections from immature into mature synapses. That’s also what happens in the developing brain when a new memory is formed from a silent synapse.
It’s unclear whether silent synapses are also prevalent in the adult human brain, though Harnett and other scientists like Sjöström think it’s likely. The researchers are now using the same techniques on human brains to find out.
Finding silent synapses in adult human brains could have implications for treating conditions such as drug addiction. Research on developing rats given cocaine suggests that drug use generates more silent synapses, which may then play a role in withdrawal symptoms. If scientists could develop a way to control the number of silent synapses, they could possibly target conditions that show abnormal levels of silent synapses.
What is clear is that silent synapses probably answer how the adult brain balances keeping old memories while making new ones, Harnett says. With this finding, “all of a sudden, solving that trade-off gets much easier.”

 A new treatment could restore some mobility in people paralyzed by strokes
A new treatment could restore some mobility in people paralyzed by strokes  What has Perseverance found in two years on Mars?
What has Perseverance found in two years on Mars?  This robot automatically tucks its limbs to squeeze through spaces
This robot automatically tucks its limbs to squeeze through spaces  Greta Thunberg’s new book urges the world to take climate action now
Greta Thunberg’s new book urges the world to take climate action now  Glassy eyes may help young crustaceans hide from predators in plain sight
Glassy eyes may help young crustaceans hide from predators in plain sight  A chemical imbalance doesn’t explain depression. So what does?
A chemical imbalance doesn’t explain depression. So what does?