
The year will not be remembered as a good one for human health. But some notable bright spots did shine through.
Ebola outbreak ends
The second biggest Ebola outbreak in history is officially over. Beginning in 2018, the virus surged in eastern Congo, infecting 3,470 people and killing about two-thirds of them (SN Online: 6/25/20). The outbreak was declared done in June thanks to an aggressive public health response involving testing, isolating sick people and contact tracing — the same measures that could slow COVID-19’s spread.
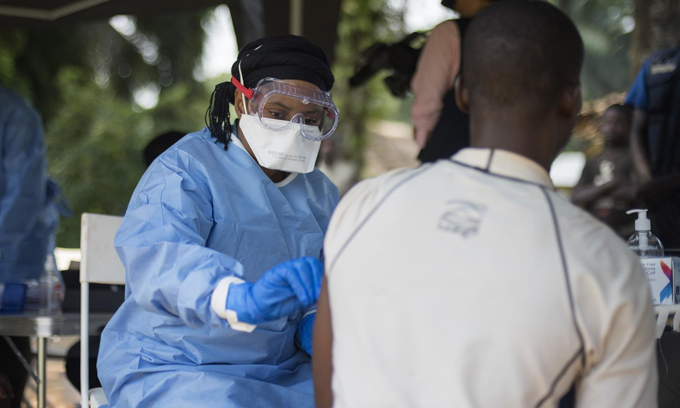
A vaccine, delivered to more than 300,000 people during the outbreak, and experimental drugs also helped. On October 14, one antibody-based treatment, Inmazeb, became the first Ebola drug approved by the U.S. Food and Drug Administration (SN Online: 10/15/20). With that approval, U.S. supplies of the drug may become more readily available for Ebola patients. (The drug’s maker, Regeneron Pharmaceuticals, is a major donor to the Society for Science & the Public, which publishes Science News.)
HIV’s elite controllers
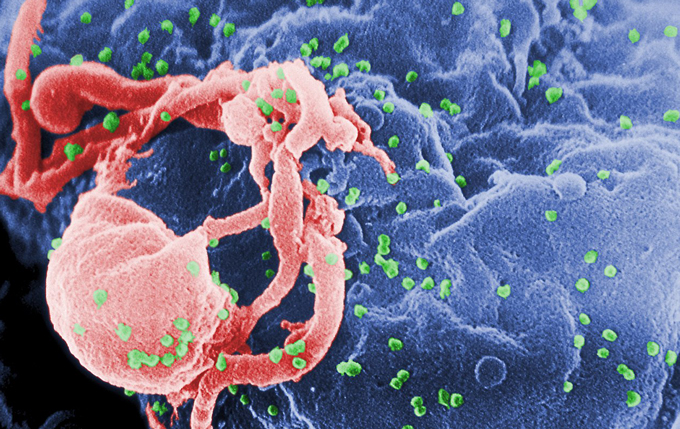
Most people with HIV take antiretroviral drugs to keep the virus in check. But in one rare person, the immune system seems to have wiped out the virus all on its own. Among over 1.5 billion blood cells taken from this once-infected person, not a single working copy of HIV turned up (SN: 9/26/20, p. 6). In another patient, researchers found only one functional copy of HIV in more than a billion blood cells. Learning how these people, part of a select group called elite controllers, fought off HIV may lead to better treatments for others.
Peanut allergy protection
In January, the FDA approved the first drug for curbing peanut allergies in children and teens (SN: 2/29/20, p. 16). Called Palforzia, the drug contains peanut proteins and is given in escalating amounts, so the body gradually learns that these proteins aren’t dangerous. The drug doesn’t go as far as eliminating peanut allergies, but it can help people tolerate an accidental peanut encounter.

 A new treatment could restore some mobility in people paralyzed by strokes
A new treatment could restore some mobility in people paralyzed by strokes  What has Perseverance found in two years on Mars?
What has Perseverance found in two years on Mars?  This robot automatically tucks its limbs to squeeze through spaces
This robot automatically tucks its limbs to squeeze through spaces  Greta Thunberg’s new book urges the world to take climate action now
Greta Thunberg’s new book urges the world to take climate action now  Glassy eyes may help young crustaceans hide from predators in plain sight
Glassy eyes may help young crustaceans hide from predators in plain sight  A chemical imbalance doesn’t explain depression. So what does?
A chemical imbalance doesn’t explain depression. So what does?